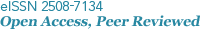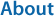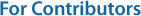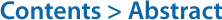JMLS 2024 June;9(1):47-52. 10.23005/ksmls.2024.9.1.47 Epub 2024 June 13
Copyright © 2024 by The Korean Society of Marine Life Science
Biological Rhythm Changes of Dominant Tidepool gunnel Pholis nebulosa in Drifting Seaweeds
Jin A Kim; Department of Convergence Study on the Ocean Science and Technology, Korea Maritime and Ocean University, Busan 49112, Korea
Min Ju Kim; Division of Marine BioScience, Korea Maritime and Ocean University, Busan 49112, Korea
Young-Su Park; Department of Nursing, Catholic University of Pusan, Busan 46252, Korea
Jun-Hwan Kim; Department of Aquatic Life Medicine, Jeju National University, Jeju 63243, Korea
Cheol Young Choi; Department of Convergence Study on the Ocean Science and Technology, Korea Maritime and Ocean University, Busan 49112, Korea Division of Marine BioScience, Korea; Maritime and Ocean University, Busan 49112, Korea
- Abstract
Light is a major external environmental factor that influences the circadian rhythm of photosynthetic organisms and various physiological phenomena, such as growth, maturation, and behavior. The number of light-reaching organisms changes depending on the season and atmospheric conditions, and the intensity and wavelength of light differ depending on the organisms inhabiting the environment. Altered light changes the circadian rhythm of fish, which is controlled by clock genes, such as period 2 (Per2), cryptochrome 1 (Cry1), and melatonin. In this study, we set the zeitgeber time (ZT; 14 light-10 dark, LD) based on the actual sunrise and sunset times and examined Per2 and Cry1 activities, levels of aralkylamine N-acetyltransferase (AANAT), and melatonin in Pholis nebulosa, a drifting seaweed species exposed to irregular light. Per2 and Cry1 levels increased during the daytime and decreased after sunset. The AANAT levels decreased during the daytime and increased during the night. Melatonin concentration was highest around midnight (ZT21, 23:30), but exhibited similar concentrations during the daytime. While the activity of Per2, Cry1, and AANAT levels exhibited a typical circadian rhythm observed in most vertebrates, melatonin concentrations did not show a significant difference between the daytime and nighttime. These findings provide insights into the circadian rhythm patterns of organisms exposed to irregular light environments, such as P. nebulosa, which differ from those of typical fish species.
Keywords: Drifting algae Circadian rhythm Clock genes Pholis nebulosa Melatonin
Correspondence to: Jun-Hwan Kim; Department of Aquatic Life Medicine, Jeju National University, Jeju 63243, Korea
- Received
- 27 December 2023;
- Revised
- 5 February 2024;
- Accepted
- 22 February 2024.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Language: Korean/English,
Full Text:

Introduction
Light is a crucial environmental factor regulating physiological processes in organisms, including fish (Cabilon and Lazado, 2019). It can induce or inhibit hormone secretion, influencing various physiological responses such as reproduction, maturation, growth, and behavior (Li et al., 2022). Also, light can be influenced by factors such as temperature, atmospheric gases, and physical environmental conditions, such as terrain and habitat, which may alter the amount of light that reaches an organism or block its transmission (Kesavan and Sagripanti, 2013; Larue et al., 2021). The intensity and wavelength of light reaching organisms can change depending on the season and external environmental factors, and these altered light conditions can affect the circadian rhythms of all organisms, including fish (Guan et al., 2022). Clock genes, including cycle 2 (Per2), cryptochrome 1 (Cry1), and melatonin, regulate circadian rhythms (Sgro et al., 2023).
Per2 and Cry1 are the major genes associated with regulating the circadian system of organisms and expression vary with exter- nal environmental conditions (Miro et al., 2023). Per2 is a hormone secreted from the suprachiasmatic nucleus (SCN) located in the middle of the brain and forms a dimer with another circadian rhythm-regulating protein, Timeless, to control the expression of clock genes (Xie et al., 2022). Similarly, Cry1 is a key gene involved in regulating circadian rhythms in vertebrates and is primarily expressed in response to light stimuli during the daytime (Liu et al., 2023). Cry breaks down the Per dimer to form a Per-Cry dimer, which plays a role in inducing changes in the nervous system and changing the circadian rhythm to control various physiological activities in various organisms, including fish (Ding et al., 2022).
Melatonin, another hormone that regulates circadian rhythms in vertebrates, is produced in the pineal gland and secreted into the bloodstream at night (Watanabe et al., 2023). In the pineal gland, the neurotransmitter 5-hydroxytryptamine (serotonin) is converted to N-acetylserotonin by the enzyme aralkylamine N-acetyltransferase (AANAT), and is subsequently catalyzed by hydroxyindole-O-methyltransferase (HIOMT) to form melatonin (Tan et al., 2021). The increased secretion of melatonin at night was attributed to the enhanced activity of AANAT, which regulates the synthesis rate of melatonin (Saha et al., 2019). In most fish species, the activity of AANAT has been reported to increase 10~100 times during the night compared to the daytime, leading to an increase in melatonin secretion into the bloodstream (Pomianowski et al., 2020; Nisembaum et al., 2021).
Regarding genetic changes and the regulation of genes related to circadian rhythms, studies have been conducted on the effects of exposure to various photoperiod environments on the growth, maturity, and physiological response of fish (Choi et al., 2019; Mazur et al., 2022). However, it is difficult to find studies that investigate the biological rhythms of fish exposed to irregular light changes due to external natural features or physical environmental conditions.
The "drifting seaweeds" noted in this study are floating seaweed masses that move along the ocean current while floating on the surface. It plays an important role as a spawning ground and hiding place in the movement, reproduction, and growth of marine life in the marine ecosystem (Dempster and Kingsford, 2004). In Korea, drifting seaweeds move from Jeju Island to the southern coast through the East China Sea and are used as a means of transportation for fish larvae or juveniles (Cho et al., 2001). Fish frequently enter and inhabit seaweed masses floating on the sea surface. However, this habitat presents an irregular light environment characterized by non-circadian variation in light ex- posure. Organisms residing there may not receive the appropriate light influence.
In this study, we investigated the effects of the topographical features of drifting seaweed on the circadian rhythm of fish by examining the influence of irregular light exposure on the habitat of the tidepool gunnel species Pholis nebulosa, which is dominant among the drifting seaweeds in the East China Sea. The experi- mental Zeitgeber time (ZT; 14 light-10 dark, LD) was determined based on natural light conditions. We examined the Per2 and Cry1 activities, AANAT levels, and melatonin concentrations at different time points to investigate the impact of the unique environment, characterized by irregular exposure to light, on the circadian rhythm of P. nebulosa.
Materials and Methods
1. Field sampling
Field sampling was conducted in the western and southern coastal areas of Korea aboard research vessel Jangmok 1, operated by the Korea Institute of Ocean Science and Technology (KIOST). Fish sampling was carried out in the waters near Wando, located at coordinates 34°13'49.22"N, 126°49'54.45"E, in the South Sea region. Seaweeds were collected by observing the sea area from the survey ship, carefully picking up seaweeds using a yard, and transferring them to a tank. The ship's engine was switched off state to not affect the creatures inside the seaweed as much as possible.
2. Experimental fish and sampling
Tidepool gunnel P. nebulosa (body length 9.2 ± 1.54 cm; mass 2.3 ± 0.70 g) were the dominant species found in the seaweeds, and they were sampled after being transferred to three 40 ℓ tanks. The water temperature was 18.6 ± 1℃, and dissolved oxygen was 3.8 ± 0.2 mg/l. At the time of sampling, the lunar phase ranged from 16.0 to 20.0, transitioning from the full moon to the last day (waning crescent). On the sampling day, sunrise occurred at 05:12 and sunset was at 19:57. Sampling was conducted at eight regular intervals, using 8:30 AM as the reference time, and was divided into day and night (14L:10D) (ZT3: 5:30, ZT6: 8:30, ZT9: 11:30, ZT12: 14:30, ZT15: 17:30, ZT18: 20:30, ZT21: 23:30, and ZT24: 2:30).
Before sampling, all fish were anesthetized with clove oil (C8392; Sigma-Aldrich, MO, USA). Tissue samples collected were imme- diately frozen on-site and stored in a -80℃ ultra-low temperature freezer until total RNA extraction. Blood was obtained from the caudal vessels using a syringe (1 ml) treated with heparin sodium. The collected blood was then separated using centrifugation (4℃, 3,000 rpm, and 10 min) to obtain plasma, which was stored in a -80℃ ultra-low temperature freezer until further analysis.
3. Analysis of Per2, Cry1 and AANAT in brain
Per2, Cry1, and AANAT levels in the brain and pituitary were analyzed using enzyme-linked immunosorbent assay (ELISA) kits (Per2, MBS108495; Cry1, MBS041774; AANAT, MBS021281; MyBioSource, San Diego, CA, USA). Brain (included pituitary) tissue was cut into small pieces and homogenized with a PBS. The homogenate was centrifuged at 3,000 rpm for 20 min. The experiment was carried out according to the manufacturer's pro- tocol using 50 μl of the supernatant as a sample. The optical density (O.D.) was measured at 450 nm using a microplate reader (PerkinElmer, Inc., Waltham, MA, USA), and the level in the sample was calculated using the standard curve.
4. Analysis of AANAT and Melatonin in plasma
The plasma melatonin concentration was analyzed using ELISA kits (Melatonin, MBS2700395; MyBioSource). Using 50 μl of the plasma as a sample, the experiment was carried out according to the manufacturer's protocol. The absorbance was read at 450 nm using a microplate reader according to the manufacturer's instruc- tions. A standard curve was created with the log of the melatonin concentration on the y-axis and absorbance on the x-axis.
5. Statistical analysis
All data were analyzed using the SPSS statistical package (version 25.0; SPSS Inc., Armonk, NY, USA). One-way analysis of variance (ANOVA) followed by Tukey's post-hoc test was used to detect statistically significant differences. A value of p < 0.05 was considered statistically significant. The measured values are ex- pressed as the mean ± standard deviations (SD).
Results
1. Per2 activity in the brain
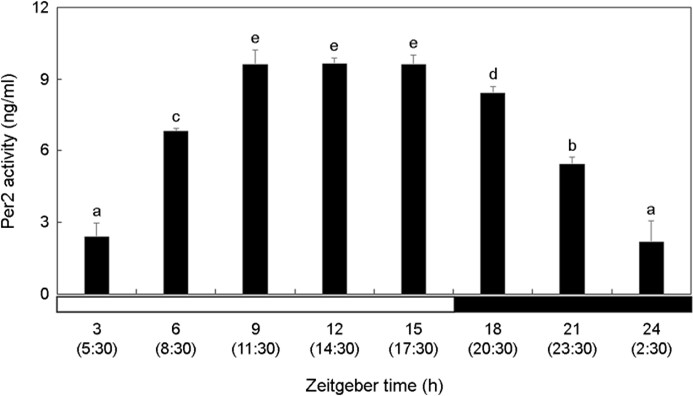
The activity of Per2 in the brain increased significantly during the photophase and decreased during the scotophase (Fig. 1). Per2 showed high activity at ZT9-ZT15 (11:30~17:30) during the daytime when there was a lot of light and the activity gradually lowered over time at night (p < 0.05). At ZT24 (2:30), Per2 activity showed the lowest value.
2. Cry1 activity in the brain
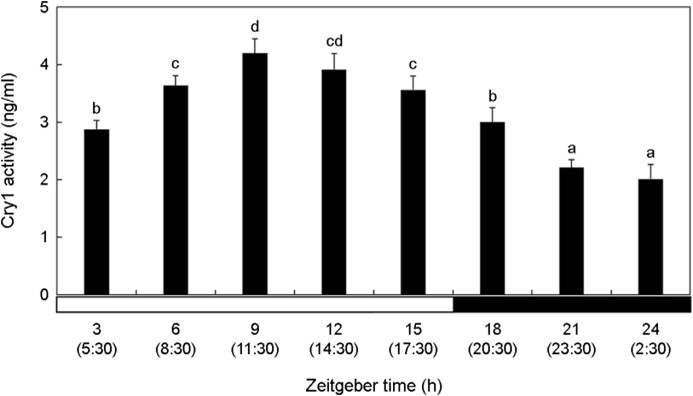
The activity of Cry1 in the brain was relatively low during the dark period compared with that during the light period (Fig. 2). Cry1 activity significantly increased at ZT3 (5:30) (p < 0.05). Cry1 activity in the brain was the lowest value at ZT21-ZT24 (23:30~ 2:30).
3. AANAT level in the brain
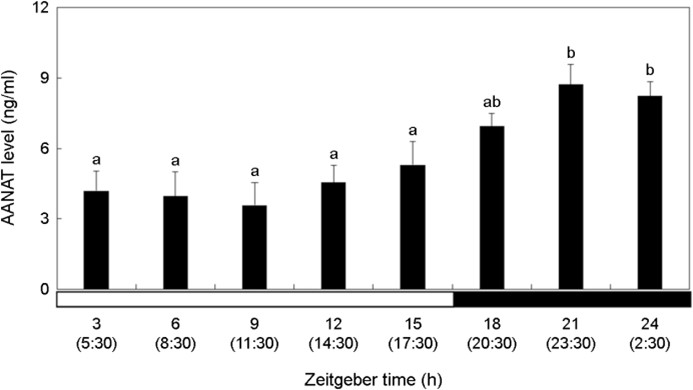
The AANAT level was relatively higher in the dark period than in the bright period (Fig. 3). AANAT showed the highest level at ZT21-ZT24 (23:30~2:30) (p < 0.05). Thereafter, it decreased during the day, and there was no significant change (p > 0.05).
4. Melatonin concentration in plasma
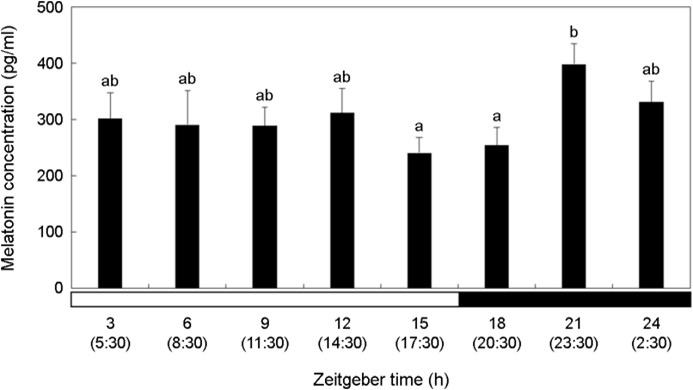
Melatonin concentration was relatively high in the dark period compared to that in the bright period (Fig. 4). Melatonin showed the highest concentration at ZT21 (23:30) (p < 0.05). It decreased after that, but the difference was not significant (p > 0.05).
Discussion
We investigated the changes in the activity of the representative clock genes, Per2 and Cry1, in P. nebulosa, which inhabit "drifting seaweeds" that are inevitably exposed to irregular light conditions due to a special external environment. Organisms are influenced by the presence or absence of light, and their biological rhythms can exhibit various physiological responses depending on changes in external environmental factors.
Kim et al. (2012) investigated the circadian rhythm changes in the olive flounder Paralichthys olivaceus, a fish species inhabiting the coastal seafloor. They reported a similar pattern in the ex- pression of Per2 and Cry1 mRNA, with both clock genes exhibited elevated expression levels during daytime and decreasing as nighttime approached. Ceinos et al. (2019) reported that when the turbot, Scophthalmus maximus was exposed to 12L:12D (12 h light, 12 h dark) and DD (24 h dark) environments, Per2 and Cry1 mRNA expression was high during the daytime in the 12L:12D environment. Comparing the DD experimental group with the 12L:12D experimental group, during the 3~9 hours of the DD group, which corresponded to the daytime in the 12L:12D group. Per2 and Cry1 mRNA expression was lower overall but still higher than at night. In the present study, Per2 and Cry1 activities of P. nebulosa began to decrease at ZT15 (17:30), which was sunset. Considering that the change in activity of P. nebulosa clock gene exposed to ZT time was also high during the day and low during the night, it can be inferred that the circadian rhythm is main- tained within the organism.
Melatonin is a key hormone that acts on the circadian rhythm of vertebrates and is regulated by AANAT. Accumulation of AANAT induces its activation and increases melatonin concentration (Tan et al., 2021). Kim et al. (2012) analyzed pineal gland AANAT and plasma melatonin concentrations in P. olivaceus exposed to a 12L:12D environment. They reported that AANAT and melatonin levels were approximately five times higher during the day than at night. When zebrafish, Danio rerio was exposed to a 12L:12D environment and a changing ZT cycle, both showed high expres- sion of AANAT2 at night (Ziv et al., 2005). Consistent with the findings of Ziv et al. (2005), we also observed a significant in- crease in AANAT levels during the night compared to the daytime. Because the investigated marine species, P. nebulosa, was exposed to irregular light conditions due to external physical factors, such as drifting seaweeds, it was anticipated that these influences might affect the expression pattern of AANAT at night. However, despite exposure to irregular light during the daytime, it appears that the circadian rhythm of P. nebulosa is not affected at night. Therefore, it can be inferred that AANAT is not influenced by irregular light exposure due to drifting seaweeds and maintains its typical pat- tern, showing higher expression during the night, as is commonly known.
Lee et al. (2021) exposed P. olivaceus to three different light cycle conditions: 12L:12D, DD, and LL (24 h light). Under the 12L:12D condition, the fish exhibited a typical circadian rhythm, with higher melatonin concentrations during the night. In the DD environment, despite ZT15 (17:30) occurring in the daytime, mela- tonin concentrations increased. In the LL environment, despite ZT18 (20:30)-ZT24 (2:30) falling in the nighttime, melatonin levels were very low. This suggests that the presence and irregularity of light may influence the expression of circadian clock genes within the organism compared to the 12L:12D condition.
In this study, P. nebulosa inhabiting drifting seaweeds showed the highest melatonin concentration at night at ZT21 (23:30). However, no significant differences in melatonin concentrations were observed between the daytime periods (ZT3-ZT12, 5:30~ 14:30) and ZT21 (23:30). This suggests that irregular light exposure within drifting seaweed prevented the fish from experiencing a normal light cycle (14L:10D), leading to an irregular response in melatonin concentrations.
Per2 and Cry1 showed high values during the daytime, and AANAT levels tended to be almost the same as those in general vertebrates, confirming the effects of certain light cycles and external habitat environments on P. nebulosa biological rhythm. However, melatonin concentration increased at night, but was similar to that at ZT3-ZT12 (5:30~14:30) during the daytime.
Thus, it was inferred that fish species inhabiting beneath float- ing drifting seaweeds on the water surface would have been irregularly influenced by light compared with typical fish species. Therefore, further research is needed to understand the effects of imbalances in biological rhythms observed in species such as P. nebulosa, which are irregularly exposed to light within drifting seaweeds, on the physiological aspects of fish.
- References
-
1. Cabillon NAR, Lazado CC. 2019. Mucosal barrier functions of fish under changing environmental conditions. Fishes 4: 2.
-
2. Ceinos RM, Chivite M, Lopez-Patino MA, Naderi F, Soengas JL, Foulkes NS, Miguez JM. 2019. Differential circadian and light-driven rhythmicity of clock gene expression and behaviour in the turbot, Scophthalmus maximus. PLoS One 14: e0219153.
-
3. Cho SH, Myoung JG, Kim JM, Lee JH. 2001. Fish fauna associated with drifting seaweed in the coastal area of Tongyeong, Korea. Trans Am Fish Soc 130: 1190-1202.
-
4. Choi CY, Kim TH, Oh YH, Min TS, Choi JY, Song JA. 2019. Effects of various LED light spectra on circadian rhythm during starvation in the olive flounder (Paralichthys olivaceus). Biol Rhythm Res 50: 355-365.
-
5. Dempster T, Kingsford MJ. 2004. Drifting objects as habitat for pelagic juvenile fish off New South Wales, Australia. Mar Freshw 55: 675-687.
-
6. Ding Y, Dong X, Feng W, Mao G, Chen Y, Qiu X, Chen K, Xu H. 2022. Tetrabromobisphenol S alters the circadian rhythm network in the early life stages of zebrafish. Sci Total Environ 806: 150543.
-
7. Guan Q, Wang Z, Cao J, Dong Y, Chen Y. 2022. The role of light pollution in mammalian metabolic homeostasis and its potential interventions: A critical review. Environ Pollut 312: 120045.
-
8. Kesavan JS, Sagripanti JL. 2013. Disinfection of airborne organisms by ultraviolet-C radiation and sunlight. Aerosol Sci Technol 17: 417-439.
-
9. Kim NN, Shin HS, Lee J, Choi CY. 2012. Diurnal gene expression of Period2, Cryptochrome1, and arylalkylamine N-acetyltrans- ferase-2 in olive flounder, Paralichthys olivaceus. Anim Cells Syst 16: 27-33.
-
10. Larue C, Sarret G, Castillo-Michel H, Pradas del Real AE. 2021. A critical review on the impacts of nanoplastics and micro- plastics on aquatic and terrestrial photosynthetic organisms. Small 17: 2005834.
-
11. Lee DW, Song JA, Park HS, Choi CY. 2021. Circadian Rhythm Disturbances Due to Exposure to Acidified Conditions and Different Photoperiods in Juvenile Olive Flounder (Paralichthys olivaceus). Ocean Sci J 56: 198-206.
-
12. Li Y, Zhu Q, Huang Y, Xu Q, Dai X, Ju C. 2022. Cloning and characterization of two types of growth hormone receptors in tomato clownfish (Amphiprion frenatus), and their expres- sion under different light spectra and photoperiods. Aquac Int 30: 1-18.
-
13. Liu Y, Wang Z, Hao H, Wang Y, Hua L. 2023. Insight into immune checkpoint inhibitor therapy for colorectal cancer from the perspective of circadian clocks. Immunology 170: 13-27.
-
14. Mazur M, Markowska M, Chadzinska M, Pijanowski L. 2022. Changes of the clock gene expression in central and per- ipheral organs of common carp exposed to constant lighting conditions. Chronobiol Int 40: 1-17.
-
15. Miro C, Docimo A, Barrea L, Verde L, Cernea S, Sojat AS, Marina LV, Docimo G, Colao A, Dentice M, Muscogiuri G. 2023. "TIME" for obesity-related cancer: The role of the circadian rhythm in cancer pathogenesis and treatment. Seminars in Cancer Biology AP 91: 99-109.
-
16. Nisembaum LG, Martin P, Lecomte F, Falcón J. 2021. Melatonin and osmoregulation in fish: A focus on Atlantic salmon Salmo salar smoltification. J Neuroendocrinol 33: e12955.
-
17. Pomianowski K, Gozdowska M, Burzyński A, Kalamarz-Kubiak H, Sokołowska E, Kijewska A, Kulczykowska E. 2020. A study of aanat and asmt expression in the three-spined stickleback eye and skin: not only "on the way to melatonin". Comp Biochem Physiol Part A Mol Integr Physiol 241: 110635.
-
18. Saha S, Singh KM, Gupta BBP. 2019. Melatonin synthesis and clock gene regulation in the pineal organ of teleost fish compared to mammals: Similarities and differences. Gen Comp Endocrinol 279: 27-34.
-
19. Sgro M, Ellens S, Kodila ZN, Christensen J, Li C, Mychasiuk R, Yamakawa GR. 2023. Repetitive mild traumatic brain injury alters central and peripheral clock gene expression in the adolescent rat. Neurobiol Sleep Circadian Rhythms 14: 100090.
-
20. Tan K, Zheng J, Liu C, Liu X, Liu X, Gao T, Song X, Wei Z, Ma F, Li C. 2021. Heterologous expression of the melatonin-related gene HIOMT improves salt tolerance in Malus domestica. Int J Mol Sci 22: 12425.
-
21. Watanabe K, Nakano M, Maruyama Y, Hirayama J, Suzuki N, Hattori A. 2023. Nocturnal melatonin increases glucose uptake via insulin-independent action in the goldfish brain. Front Endocrinol 14: 1173113.
-
22. Xie T, Guo D, Luo J, Guo Z, Zhang S, Wang A, Wang X, Wang X, Cao W, Su L, Guo J, Huang R, Xiao Y. 2022. The Relationship between HIF1α and clock gene expression in patients with Obstructive Sleep Apnea. Nat Sci Sleep 14: 381.
-
23. Ziv L, Levkovitz S, Toyama R, Falcon J, Gothilf Y. 2005. Functional development of the zebrafish pineal gland: light-induced expression of period2 is required for onset of the circadian clock. J Neuroendocrinol 17: 314-320.