JMLS 2023 June;8(1):78-86. 10.23005/ksmls.2023.8.1.78 Epub 2023 June 16
Copyright © 2023 by The Korean Society of Marine Life Science
Acute and Chronic Effects of Nanoplastics on the Water Flea Moina macrocopa
Md. Niamul Haque; Department of Marine Science, College of Natural Sciences, Incheon National University, Incheon 22012, Korea; Research Institute of Basic Sciences, Incheon National University, Incheon 22012, Korea
Jaehee Kim; Department of Marine Science, College of Natural Sciences, Incheon National University, Incheon 22012, Korea
Jae-Sung Rhee; Department of Marine Science, College of Natural Sciences, Incheon National University, Incheon 22012, Korea; Research Institute of Basic Sciences, Incheon National University, Incheon 22012, Korea; Yellow Sea Research Institute, Incheon 22012, Korea
- Abstract
Here, upon acute (96 h) and chronic (14 days) exposure, ingestion of polystyrene NPs (100 nm) and physiological, biochemical, and cholinergic modulations were analyzed in the water flea Moina macrocopa exposed to different concentrations (0.001, 0.01, 0.1, 1, 5, 10, 50, 100, and 500 μg l-1). Exposed NPs were observed in the internal organs (e.g., digestive tract and foregut) of the water flea. Chronic exposure to the relatively high concentrations resulted in significant decreases in survival, body length, and the total number of molts, whereas reproduction parameter was not affected. Significant increase in oxidative stress biomarker (malondialdehyde) and decrease in the intracellular content of endogenous antioxidant (glutathione) and enzymatic activity of antioxidant enzymes (glutathione peroxidase, glutathione reductase, catalase, superoxide dismutase, and glutathione S-transferase) were detected in response to relatively high concentrations of NPs. Transcriptional expression of the hsp70 gene was increased in response to relatively high concentrations of NPs, whereas acetylcholinesterase activity was lowered by the same concentrations of NPs. Taken together, NPs exposure would be a significant modulator on physiological and biochemical metabolism of water flea.
Keywords: Water flea Polystyrene Nanoplastics Chronic toxicity Oxidative stress
Correspondence to: Jae-Sung Rhee; Department of Marine Science, College of Natural Sciences, Incheon National University, Incheon 22012, Korea
- Received
- 2 December 2022;
- Revised
- 13 December 2022;
- Accepted
- 1 May 2023.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Plastics are ubiquitous in the aquatic environment due to the increased production and extensive usage (El Hadri et al., 2020). Plastic debris has been highlighted as emerging pollutants in the aquatic environment due to their persistency and potential toxicity. Since nano- and micro-sized plastics (NPs and MPs) have been detected globally, potential harmful effects of the plastic debris on aquatic organisms have received significant attention (Lambert and Wagner, 2016; Hartmann et al., 2019). NPs (1 to 1,000 nanometer in diameter) can be originated from the frag- mentation, microbial degradation, and weathering of numerous plastic debris and other sources from nano-sized plastics applied in commercial products (e.g., adhesives, paints, and shampoos) (Koelmans et al., 2015). NPs pose a unique threat to aquatic organisms, as its size is comparable to nanoparticles that can be transported across cell membranes (Rossi et al., 2014). NPs can be ingested by zooplanktons through filter feedings activity, causing various adverse effects, including growth inhibition, oxidative stress induction, reproductive disorder, and neurotransmission dysfunction (Rist et al., 2017; Varó et al., 2019; Zhang et al., 2019). Due to the unique characteristics of NPs such as small size, large surface area, and higher bioavailability, they can be toxic to aquatic organisms (Lehner et al., 2019)). Buoyancy and persistency of NPs finally leads to their global distribution in numerous aquatic ecosystems in- cluding Polar regions and even atmosphere (ter Halle et al., 2017; Gigault et al., 2018; Aeschlimann et al., 2022; Materić et al., 2022). Finally, global presence of NPs in aquatic environments and their detrimental effects make them one of the most critical problems that need to be addressed urgently in aquatic animals.
Decreased growth, molting, survival, and reproduction have been observed in zooplankton exposed to NPs (Besselin et al., 2014; Bergami et al., 2016; Cui et al., 2017; Eom et al., 2020, 2021; Lee et al., 2021; Yoo et al., 2021). A recent study showed that NPs induce oxidative stress in Daphnia pulex and significantly modulate mRNA expressions of stress defense genes (i.e., heat shock proteins) (Liu et al., 2019). In general, oxidative stress is in- duced by an imbalance between the levels of intracellular reactive oxygen species (ROS) and antioxidants in animals (Lushchak, 2011). ROS can be produced by mitochondria in cells and excessive ROS can damage DNA, proteins, and lipids (Regoli and Giuliani, 2014). The attack of ROS on the lipids of cellular membranes generates malondialdehyde (MDA). MDA is the final peroxidation product of polyunsaturated fatty acids, and its modulation is commonly known as a strong biomarker of antioxidant status in cells and animals. Antioxidants can neutralize ROS and decrease the risk of oxidative damage (Sies, 1991). GSH is an important antioxidant and intracellular thiol that participates in the detoxification of xenobiotics and serves as a cofactor in isomerization reactions (Lushchak, 2011). Of antioxidant enzymes, superoxide dismutase (SOD) detoxifies superoxide anion (O-2) and converts it into H2O2 and O2. Catalase (CAT) and glutathione peroxidase (GPx) detoxify H2O2 into H2O and O2. Glutathione reductase (GR) converts oxi- dized GSH (GSSG) to reduced glutathione (GSH) using NADPH as a cofactor. Glutathione S-transferase (GST) catalyzes the con- jugation of GSH to endogenous and exogenous xenobiotics (Sies, 1991; Regoli and Giuliani, 2014). The key role of acetylcholin- esterase (AChE) enzyme is the hydrolysis of acetylcholine (Ach, a mediator of neurotransmission in cholinergic synapses) into choline and acetic acid (Lin et al., 2019). Increase or decrease in AChE enzyme activity has been used to understand the potential neuro- toxic effects of plastic debris in different organisms (Varó et al., 2019). In the case of heat shock protein 70 (hsp70), its increase is commonly regarded as a stress response in organisms. Con- sequently, the measurement of transcriptional expression of hsp70 has been used to study the potential toxic effects of NPs (Liu et al., 2019; Varó et al., 2019).
Polystyrene is a plastic polymer and is commonly found in aquatic environments (Eom et al., 2022). The aging processes of NPs may modify colloidal stability and oxygen-containing func- tional groups [e.g., carboxyl (CO) and hydroxyl (OH) groups] on the surface of NPs and this process can induce toxic effects on aquatic animals (Liu et al., 2019). In this study, acute (96 h) and chronic (14 days) exposure to polystyrene NPs was conducted in a water flea. The water flea Moina macrocopa as a model for toxicity test has several experimental advantages, including small size, easy maintenance, short life cycle, sensitivity to contaminants, and wide geographical distribution around the world (Kim and Rhee, 2021; Liu et al., 2022). Their habitats in the upper layer of water bodies allow them to be more susceptible to small particles such as NPs. By transporting energy from phytoplankton (pro- ducers) to higher trophic levels (consumers), zooplankton, including water fleas, play a key role in the functioning of aquatic food webs (Liu et al., 2022). While the ingestion of NPs by water flea species has received extensive attention, little is known about their harmful effects of NPs on biochemical components. Therefore, this study aimed to investigate NPs ingestion, changes in physiological features, and modulations in molecular and biochemical markers in water flea exposed to NPs. Our study will contribute to the comprehensive insight into the understanding of the detrimental effects of NPs on zooplankton.
Materials and Methods
1. Water flea culture
The cladoceran M. macrocopa has been continuously cultured in an aquaculture system under static-renewed conditions at Incheon National University (Incheon, South Korea). The water flea were cultured at 20 individuals per 800 ml of culture medium (pH 7.5) in a 1 ℓ glass beaker at 20℃ under a light and dark (LD) photoperiod of 14:10 h in a fully defined Elendt M4 medium and replaced twice a week. The medium was prepared with distilled water after filtration (Millipore, Bedford, MA, USA). The animals were fed once daily with a mixture of green alga Chlorella vulgaris (2 × 105 cells ml-1) and baker's yeast.
2. Nanoplastics
To confirm the ingestion of plastic debris by the water flea, 0.1 μm of fluorescent spherical particles based on melamine resin [carboxylate-modified; fluorescein isothiocyanate (FITC)-marked; analytical standard] was purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). The mean diameter was 0.1 μm, and the cali- brated particle diameter was 0.091~0.118. The fluorescence of the polystyrene NPs was measured using a Nikon SMZ25 stereo- microscope (Nikon, Japan) and pictures were captured with a high-resolution camera linked to the measurement tools of the Nikon imaging software (NIS-Element Analysis D 4.20.00). For the chronic exposure, 0.1 μm of non-functionalized spherical poly- styrene NPs was purchased as an aqueous suspension (2.5 mg ml-1 in distilled water) from Sigma-Aldrich, Inc. The mean diameter was 0.1 μm, and the calibrated particle diameter was 0.093~0.110 μm. The suspension was mixed using a vortex before dilution and exposure and the surfactant (Tween 20, Sigma-Aldrich, Inc.; 0.01%) was used during the dilution procedure. Working solutions at designated nominal concentrations were prepared by diluting a stock solution in Elendt M4 medium.
3. Chronic exposure
Overall, chronic exposure and measurement of reproductive output was conducted based on the standard protocol for water flea (OECD, 2012). Ten neonates (< 24 h) were individually ex- posed to different concentrations (0.001, 0.01, 0.1, 1, 10, 50, 100, and 500 μg l-1) of polystyrene NPs for 14 days in a semi-static culture condition. The culture medium was changed twice a week with the addition of the same concentration of polystyrene NPs. The produced offspring were immediately transferred to daily. They were fed once daily. The water fleas were monitored under a Nikon SMZ25 stereomicroscope (Nikon) and their movements were recorded as a video file with a high-resolution camera linked to the stereomicroscope. Body length and reproductive parameters were measured using the measurement tools of the Nikon imaging software (NIS-Element Analysis D 4.20.00).
4. Measurement of biochemical parameters
Since high mortality was observed in the water flea exposed to 500 μg l-1 on day 7, to obtain an early signal, acute biochemical responses were analyzed for 96 h upon 100 μg l-1. Approximately, 100 water fleas were prepared for each concentration to analyze modulations of the biochemical parameters on polystyrene NPs. They were randomly separated into three groups (30~33 individ- uals per replicate) as a triplicate. After exposure to different con- centrations (0.001, 0.01, 0.1, 1, 10, 50, and 100 μg l-1) of polystyrene NPs, individuals for each group were pooled for the experiment. Using a Teflon homogenizer, each sample was homogenized in a cold buffer (20 mM Tris buffer, 100 μM benzamidine, 2 μM aprotinin, 150 mM NaCl, 10 mM β-mercaptoethanol, and 20 μM leupeptin). The samples were centrifuged at 30,000 ×g and 4℃ for 30 min, and the collected supernatant was denatured at 75℃ for 15 min.
Thiobarbituric acid reactive substances were analyzed at an excitation wavelength of 535 nm using a Varioskan Flash spectro- photometer (Thermo Fisher Scientific, Tewksbury, MA, USA). The content of MDA-the major product of lipid peroxidation-was measured using a calibration curve prepared based on malondi- aldehyde bis (dimethyl acetal) (Sigma-Aldrich Inc.) and expressed as nanomole of MDA per microgram of protein. The protein con- tent was determined according to the Bradford method (Bradford, 1976).
The GSH content was determined using the Glutathione Assay Kit (Catalog No. CS0260; Sigma-Aldrich Inc.). The samples from each treatment were cleaned with 0.9% NaCl. The clean samples were homogenized in trichloroacetic acid (1:4, w/v) using a Teflon homogenizer and centrifuged at 3,000 ×g and 4℃ for 10 min. The supernatant was collected, and the GSH content of the super- natant was measured at 420 nm according to the manufacturer's protocol using a Varioskan Flash spectrophotometer (Thermo Fisher Scientific). For measuring the total GSH content, standard curves were obtained with GSH equivalents of 0, 150, and 350 μM.
The enzymatic activities of glutathione peroxidase (GPx) and glutathione reductase (GR) were calculated using the Glutathione Peroxidase Cellular (Sigma-Aldrich, Inc.) and Glutathione Reductase (Sigma-Aldrich, Inc.) Assay Kits, respectively. CAT and SOD activity in the pooled samples from each treatment was analyzed with the CAT (Catalog No. CAT100; Sigma-Aldrich Inc.) and SOD (Catalog No. 19160; Sigma-Aldrich Inc., Chemie, Switzerland) assay kits. Using a Teflon homogenizer, the samples were homogenized in ice cool buffer (1:4, w/v) containing 0.5% Triton X-100 and 0.25 M sucrose (pH 7.5) and then centrifuged at 3,000 ×g and 4℃ for 30 min. The supernatant was collected, and its absorbance was measured at 520 nm to determine CAT activity and at 440 nm to determine SOD activity using the Varioskan Flash spectropho- tometer (Thermo Fisher Scientific) at 25℃. The total protein content was determined using the Bradford method.
The total GST activity was evaluated according to our previous study on the water flea (Kim and Rhee, 2021). The samples were homogenized using a Teflon homogenizer with cold buffer [1:4, w/v; 0.25 M sucrose, 10 mM Tris, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.2 mM dithiothreitol (DTT), and 0.1 mM phenyl- methylsulfonyl fluoride (PMSF), pH 7.4] and centrifuged at 10,000 g for 10 min at 4℃. The supernatant containing the enzyme was collected for enzymatic analysis with 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. GST activity was monitored at 340 nm at 25℃. Enzymatic activities were normalized by the total protein concentration in the samples and quantified with the Bradford method. Enzymatic activity was represented as a U per mg protein.
AChE activity was determined using acetylthiocholine iodide (ATCh) and 5,5'-dithiobis (2-nitrobenzoic acid) (DTNB) (Sigma-Aldrich Inc.). The samples were mixed with potassium phosphate buffer (100 mM, pH 7.4) and then centrifuged at 5,000 ×g and 4℃ for 1 min. The supernatant was collected to analyze AChE activity. Subsequently, 100 μl of the supernatant was transferred to a 3 ml cuvette and mixed with 1.3 ml of phosphate buffer (0.1 M, pH 8.0). Substrates, 10 μl of ATCh (0.075 M) and 50 μl of DTNB (0.01 M) were added. At 25℃, total AChE activity was measured using a blank without the sample and a blank without ATCh at 412 nm with the Varioskan Flash spectrophotometer (Thermo Fisher Scientific). The enzyme activity was normalized to the total protein content of the supernatant and expressed relative to the control. AChE activity was expressed in micromoles of hydrolyzed acetylcholine chloride per minute per microgram of protein.
5. Statistics
SPSS version 17.0 (SPSS Inc., Chicago IL, USA) was used for statistical analyses. All data were expressed as mean and standard deviation. Significant differences among the experimental groups were determined using one-way analysis of variance (ANOVA) followed by post-hoc Tukey and Dunnett's multiple comparison tests. The significance level was set at p < 0.05.
Results
1. Nanoplastics ingestion
By observing fluorescence, time-course ingestion and excretion of NPs was confirmed in the water flea (Fig. 1). NPs movements were mainly detected in the digestive systems (i.e., foregut and digestive tract) for 5 h.

2. Physiological responses to chronic exposure
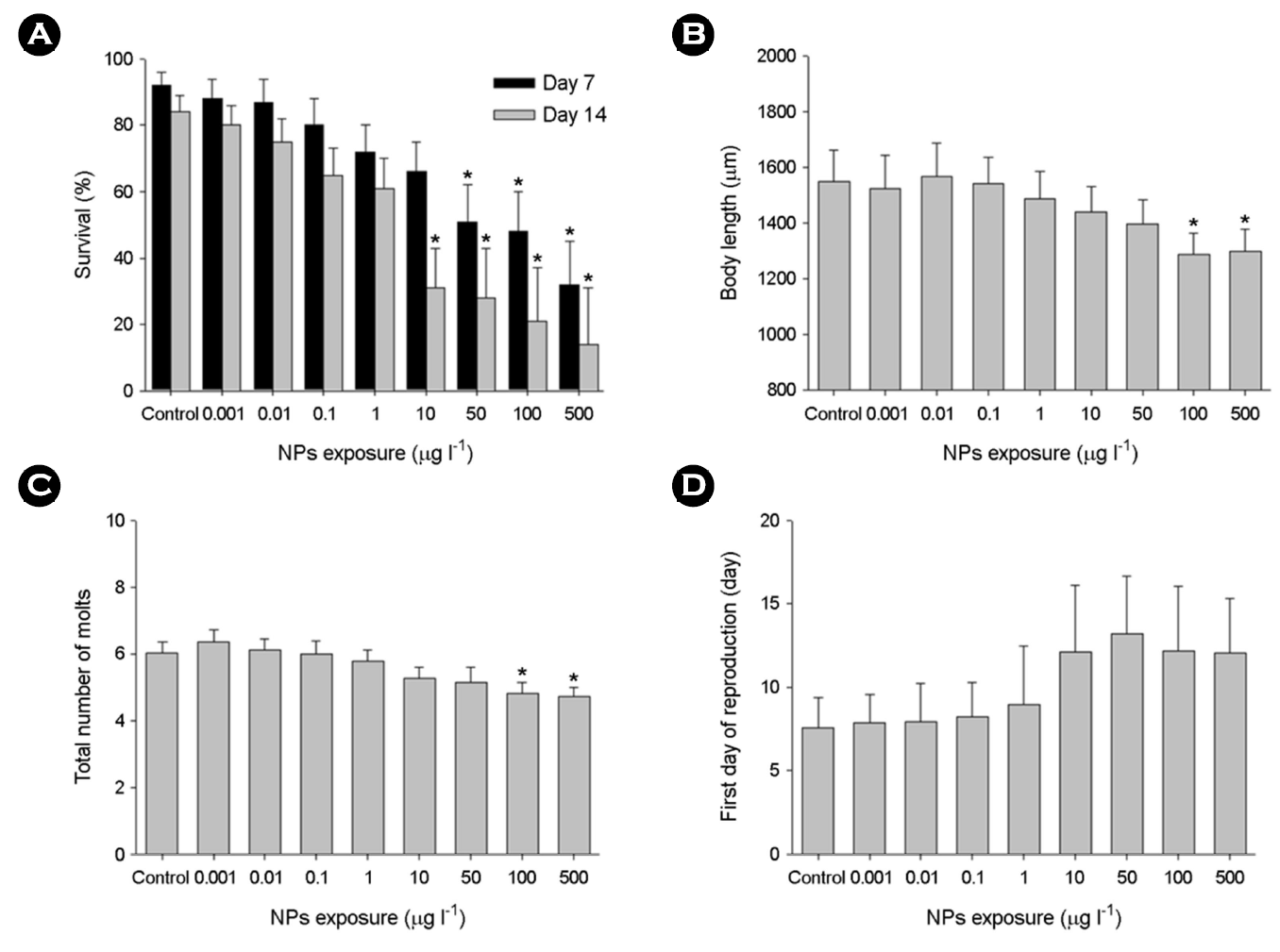
The percentage of water fleas that survived in response to different concentrations of NPs was measured at days 7 and 14 (Fig. 2A). Substantial impacts on the survival of water fleas by higher NPs concentrations (50~500 μg l-1) were confirmed at days 7 and 14 (p < 0.05). Mortality of water fleas was clearly time- and concentration-dependent.
Upon exposure to 100 and 500 μg l-1 NPs, the body length (Fig. 2B) and the total number of molts (Fig. 2C) were significantly reduced in a concentration-dependent manner for 14 days (p < 0.05). The intermolt durations were significantly extended upon the higher concentrations of NPs (100~500 μg l-1). The first day of reproduction in water fleas was not significantly affected by NPs (p > 0.05) (Fig. 2D).
3. Biochemical responses
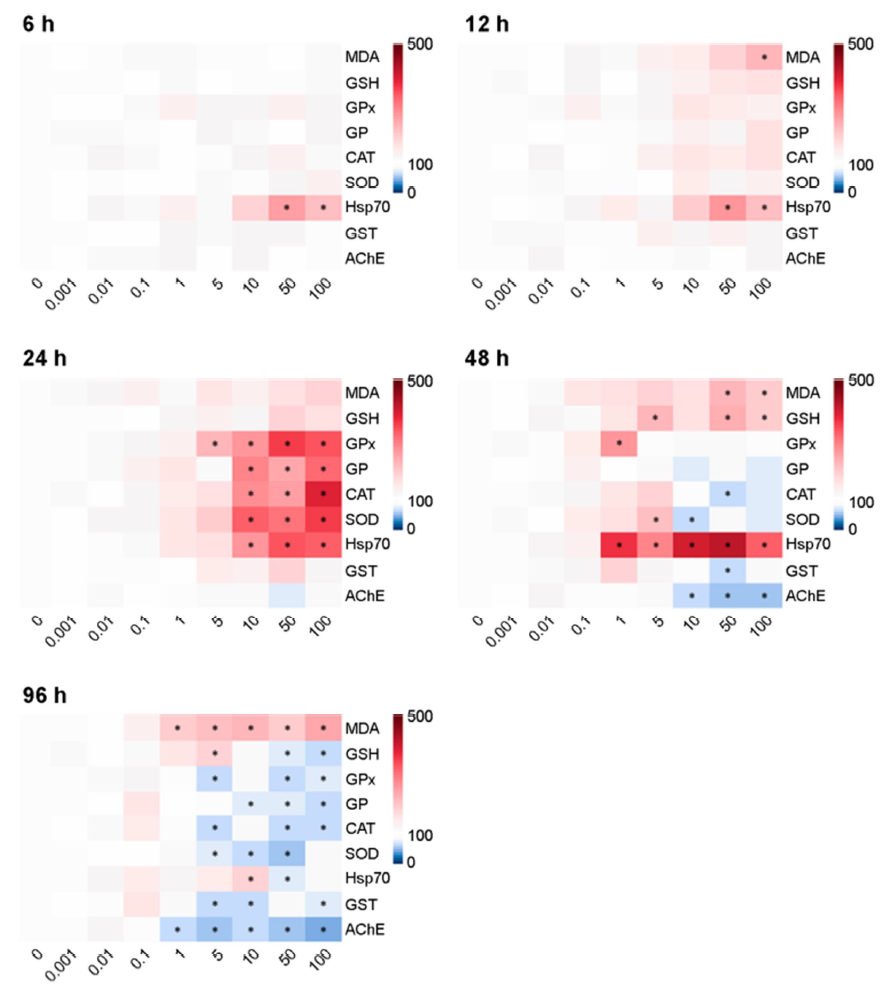
The patterns of biochemical responses differed with NPs con- centrations and exposure period. The statistical significance of all parameters on each biochemical result is shown using a heat map (Fig. 3). The levels of MDA in water fleas were significantly in- creased depending on microplastic concentrations and exposure duration (p < 0.05). The levels of GSH were significantly increased after 48 h, but the level was significantly depleted at 96 h in response to relatively higher concentrations of NPs (50~100 μg l-1) (p < 0.05). At relatively higher concentrations of NPs, enzyme activities of GPx and GR proteins were significantly elevated at 24 h, while their levels were significantly lowered at 96 h (p < 0.05). The enzymatic activities of CAT and SOD proteins were sig- nificantly higher at 24 h, but their levels were significantly reduced at 96 h in response to relatively higher NPs concentrations (p < 0.05). The enzymatic activities of GST and AChE proteins were significantly decreased by relatively higher concentrations of NPs at 48 and 96 h. Transcriptional expression of the hsp70 gene was significantly elevated for 48 h (p < 0.05), but the level was slightly down-regulated at 96 h.
Discussion
Analysis of fluorescence image of the foregut and digestive tract indicates that NPs are clearly ingested in water fleas. Differences in filter-feeding capability (e.g., leg movement), intake pathways (e.g., filtration, penetration, and absorption), and life stage could be reasons for the variation in NPs ingestion in aquatic animals (Cole et al., 2013; Liu et al., 2019). An increase in NPs uptake with the increase in exposure time was recorded in the water flea Daphnia magna when they exposed to 10 μg ml-1 for 6 h (Nasser and Lynch, 2016). In Daphnia pulex, 75 nm-sized NPs were clearly ingested after exposure to 2 mg l-1 for 24 h (Liu et al., 2019). Retention in the internal body might lead to NPs mobility across trophic levels.
Several studies have reported that the survival of cladocerans can be influenced by NPs (Cui et al., 2017; Liu et al., 2019; Yoo et al., 2021). In this study, relatively higher concentrations of NPs and their exposure period significantly reduced the survival rates of the water fleas, indicating that concentrations and exposure period can induce a potential toxic impact on cladoceran survival. Comparable results were reported in the water flea Daphnia pulex, as 100% mortality was observed in response to 400 mg l-1 of polystyrene NPs for 48 h, whereas 4% mortality was recorded after exposure to 10 mg l-1 (Liu et al., 2019). Exposure to NPs can directly induce gut blockage and reduce feeding rates as shown in water flea (Rist et al., 2017). This could be one possible reason for the significant mortality observed at days 7 and 14.
The body length of the water fleas was significantly inhibited by higher concentrations of NPs (50~100 μg l-1), suggesting that NPs induce notable harmful effects on growth. Previously, the exposure of Daphnia magna to polystyrene NPs with a con- centration of 0.5 mg l-1 for 7 days and 0.1 mg l-1 for 14 days significantly decreased their body length (Liu et al., 2019).
Molting is energy consuming and hormone-controlled physio- logical phenomenon (Bergami et al., 2016). In Cladocera, growth and molting are intrinsically interconnected as the body length mainly depends on the number of molt. Our results indicate that exposure of water flea to relatively higher concentrations of NPs modulates the molting process of the water fleas and subsequently affects their growth rates. A strong correlation between NPs and modulation of molting as well as growth inhibition was suggested in the aquatic crustaceans, Artemia franciscana (Bergami et al., 2016) and Daphnia pulex (Auffan et al., 2013). No significant alteration on the first reproduction day was observed in this study, although delayed reproduction to increased exposure duration was shown in the marine rotifer Brachionus koreanus exposed to NPs (Jeong et al., 2021).
Treatment of NPs can induce oxidative stress in aquatic organ- isms (Trevisan et al., 2022). The significant increase in MDA level after 96 h exposure indicates NPs-induced intracellular ROS and oxidative damage through lipid peroxidation. Similarly, 0.05 and 0.5 μm polystyrene NPs (1 and 10 mg l-1) showed higher MDA levels for 48 h in Diaphanosoma celebensis (Yoo et al., 2021). NPs-triggered lipid peroxidation and oxidative stress were evident in response to 14 days exposure to polystyrene NPs (50 nm) in Artemia franciscana, while 48 h exposure did not induce lipid peroxidation (Varó et al., 2019). A nonenzymatic antioxidant par- ameter, GSH is responsible for maintaining the cellular redox state and protecting cells from oxidative stress (Regoli and Giuliani, 2014). In the present study, GSH content was significantly increased after NPs exposure at 48 h, but the level was significantly decreased at 96 h, indicating time-dependent capacity of the antioxidant defense component. Increased GSH levels suggest the induction of oxidative stress and decreased GSH levels indicate cellular vul- nerability to oxidative stress as the thiol-group of GSH maintains the cellular ROS, manifesting the equilibrium balance of GSH and glutathione disulfide (GSSG) ratio (Lushchak, 2011). Similarly, a significant modulation in GSH levels by NPs was observed in aquatic organisms (He et al., 2020; Fan et al., 2022; Han et al., 2022).
We observed that the enzymatic activity of the antioxidant defense system (i.e., GPx, GR, CAT, SOD, and GST) were increased patterns for 48 h of exposure to NPs, while their activity was significantly decreased at 96 h. These antioxidant components protect organisms from numerous environmental stressors. In aquatic organisms, the levels of antioxidant enzymes have been shown to be modulated by NPs with induction of intracellular ROS and oxidative stress (Liu et al., 2019; Yoo et al., 2021; De Felice et al., 2022). For example, the time-dependent modulation in enzymatic activities was detected in the brine shrimp Artemia franciscana exposed to polystyrene NPs (Varó et al., 2019). Our results indicate that the activation of antioxidant enzymes, including GPx, GR, CAT, SOD, and GST, is associated with a higher defense mechanism against NPs-induced oxidative stress. In fact, generation of free radicals and subsequent key events of oxidative stress such as lipid peroxidation, DNA damage, and activation of antioxidant defense system have been consistently studied in aquatic animals (Hu and Palić, 2020). Decreased antioxidant enzymes measured at 96 h indicate suppression of antioxidant defense and higher vulnerability to oxidative damage, whereas the antioxidation en- zymes may not be able to maintain the balance between the generation of ROS and the antioxidant defense. Overall, our findings along with those of other studies, represent that the exposure period of NPs is a crucial factor in determining the degree of their toxicity.
Hsp70 is a stress-responsive protein and vital for maintaining protein homeostasis. Hsp70 is induced when cells are exposed to various environmental stressors (Guo et al., 2007; Rhee et al., 2009). In this study, the increase in hsp70 transcripts indicates that NPs affected protein homeostasis and subsequently induced the transcriptional expression of hsp70 to prevent potential damage on protein metabolism (Kiang and Tsokos, 1998). Exposure of D. magna (Liu et al., 2019) and Artemia (Varó et al., 2019) to NPs significantly upregulated mRNA expression of hsp70 as shown in this study.
The modulation in AChE activity in response to NPs has been found in Artemia species (Varó et al., 2019; Eom et al., 2020). The significant decrease in AChE activity after acute exposure indicates that NPs produced potential neurotoxicity in water fleas. The inhibition of AChE activity could be neurotransmitter-blocking effects of NPs (Lin et al., 2019). Previously, it was shown that in the brine shrimp (Artemia franciscana), inhibition of AChE activity by NPs was induced by 14-day exposure, while no significant effect was observed by short-term exposure (48 h) (Varó et al., 2019). No significant alteration in AChE activity during the short-term exposure (6 to 24 h) observed in this study may be related to the tolerance (homeostatic ability to set up the detoxification mechanisms) of the water fleas against ROS and oxidative stress (Varó et al., 2019).
In summary, the water fleas clearly ingested NPs and were sensitive to acute and chronic exposure to NPs. Physiological par- ameters (e.g., survival rate, growth, and the total number of molts) were dose-dependently reduced. Oxidative stress was induced by acute exposure to NPs, as a reduction in the activity of the antioxidant enzymes and an increase in lipid peroxidation were observed. NPs modulated mRNA expression of hsp70 and AChE activity in the water fleas, suggesting stress defense and neuro- logical impairment after acute exposure. Overall, the exposure period and NPs concentrations were key factors for the detrimental effects of NPs. Future studies on NPs toxicity should also focus on understanding the toxic mechanisms and ecological hazards in a wide array of aquatic organisms.
- References
-
1. Aeschlimann M, Li G, Kanji ZA, Mitrano DM. 2022. Microplastics and nanoplastics in the atmosphere: the potential impacts on cloud formation processes. Nat Geosci 15: 967-975.
-
2. Auffan M, Bertin D, Chaurand P, Pailles C, Dominici C, Rose J, Bottero JY, Thiery A. 2013. Role of molting on the biodistri- bution of CeO2 nanoparticles within Daphnia pulex. Water Res 47: 3921-3930.
-
3. Bergami E, Bocci E, Vannuccini ML, Monopoli M, Salvati A, Dawson KA, Corsi I. 2016. Nano-sized polystyrene affects feeding, behavior and physiology of brine shrimp Artemia franciscana larvae. Ecotoxicol Environ Saf 123: 18-25.
-
4. Besseling E, Wang B, Lürling M, Koelmans AA. 2014. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol 48: 12336-12343.
-
5. Bradford MM. 1976. A rapid and sensitive method for the quan- titation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
-
6. Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS. 2013. Microplastic ingestion by zooplankton. Environ Sci Technol 47: 6646-6655.
-
7. Cui R, Kim SW, An YJ. 2017. Polystyrene nanoplastics inhibit re- production and induce abnormal embryonic development in the freshwater crustacean Daphnia galeata. Sci Rep 7: 12095.
-
8. De Felice B, Sugni M, Casati L, Parolini M. 2022. Molecular, bio- chemical and behavioral responses of Daphnia magna under long-term exposure to polystyrene nanoplastics. Environ Int 164: 107264.
-
9. El Hadri H, Gigault J, Maxit B, Grassl B, Reynaud S. 2020. Nano- plastic from mechanically degraded primary and secondary microplastics for environmental assessments. Nano Impact 17: 100206.
-
10. Eom H-J, Nam S-E, Rhee J-S. 2020. Polystyrene microplastics induce mortality through acute cell stress and inhibition of cholinergic activity in a brine shrimp. Mol Cell Toxicol 16: 233-243.
-
11. Eom H-J, Haque MN, Lee S, Rhee J-S. 2021. Exposure to metals premixed with microplastics increases toxicity through bio- concentration and impairs antioxidant defense and cholinergic response in a marine mysid. Comp Biochem Physiol C Toxicol Pharmacol 249: 109142.
-
12. Eom H-J, Lee N, Yum S, Rhee J-S. 2022. Effects of extremely high concentrations of polystyrene microplastics on asexual repro- duction and nematocyst discharge in the jellyfish Sanderia malayensis. Sci Total Environ 807: 150988.
-
13. Fan W, Yang P, Qiao Y, Su M, Zhang G, 2022. Polystyrene nano- plastics decrease molting and induce oxidative stress in adult Macrobrachium nipponense. Fish Shellfish Immunol 122: 419-425.
-
14. Gigault J, ter Halle A, Baudrimont M, Pascal P-Y, Gauffre F, Phi T-L, El Hadri H, Grassl B, Reynaud S. 2018. Current opinion: what is a nanoplastic? Environ Pollut 235: 1030-1034.
-
15. Guo S, Wharton W, Moseley P, Shi H. 2007. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 12: 245-254.
-
16. Han M, Gao T, Liu G, Zhu C, Zhang T, Sun M, Li J, Ji F, Si Q, Jiang Q. 2022. The effect of a polystyrene nanoplastic on the intes- tinal microbes and oxidative stress defense of the freshwater crayfish, Procambarus clarkii. Sci Total Environ 833: 155722.
-
17. Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, E. daugaard AS, Rist S, Karlsson T, Brennholt N, Cole M, Herrling MP, C. Hess MC, Ivleva NP, Lusher AL, Wagner M. 2019. Are we speaking the same language? Recommen- dations for a definition and categorization framework for plastic debris. Environ Sci Technol 53: 1039-1047.
-
18. He Y, Li J, Chen J, Miao X, Li G, He Q, Xu H, Li H, Wei Y. 2020. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci Total Environ 723: 138180.
-
19. Hu M, Palić D. 2020. Micro- and nano-plastics activation of oxi- dative and inflammatory adverse outcome pathways. Redox Biol 37: 101620.
-
20. Jeong CB, Kang HM, Byeon E, Kim MS, Ha SY, Kim M, Jung JH, Lee JS. 2021. Phenotypic and transcriptomic responses of the rotifer Brachionus koreanus by single and combined expos- ures to nano-sized microplastics and water-accommodated fractions of crude oil. J Hazard Mater 416: 125703.
-
21. Kiang JG, Tsokos GC. 1998. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther 80: 183-201.
-
22. Kim J, Rhee J-S. 2021. Biochemical and physiological responses of the water flea Moina macrocopa to microplastics: a multi- generational study. Mol Cell Toxicol 17: 523-532.
-
23. Koelmans AA, Besseling E, Shim WJ. 2015. Nanoplastics in the aquatic environment. Critical review. In: Bergmann M, Gutow L, Klages M. (Eds.), Marine Anthropogenic Litter. Springer, Cham, 325-340.
-
24. Lambert S, Wagner M. 2016. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145: 265-268.
-
25. Lee D-H, Lee S, Rhee J-S. 2021. Consistent exposure to micro- plastics induces age-specific physiological and biochemical changes in a marine mysid. Mar Pollut Bull 162: 111850.
-
26. Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B. 2019. Emergence of nanoplastic in the environment and possible impact on human health. Environ Sci Technol 53: 1748-1765.
-
27. Lin W, Jiang R, Hu S, Xiao X, Wu J, Wei S, Xiong Y, Ouyang G. 2019. Investigating the toxicities of different functionalized poly- styrene nanoplastics on Daphnia magna. Ecotoxicol Environ Saf 180: 509-516.
-
28. Liu Q, Liu L, Huang J, Gu L, Sun Y, Zhang L, Lyu K, Yang Z. 2022. The response of life history defense of cladocerans under predation risk varies with the size and concentration of micro- plastics. J Hazard Mater 427: 127913.
-
29. Liu Y, Hu Y, Yang C, Chen C, Huang W, Dang Z. 2019. Aggregation kinetics of UV irradiated nanoplastics in aquatic environments. Water Res 163: 114870.
-
30. Liu Z, Yu P, Cai M, Wu D, Zhang M, Huang Y, Zhao Y. 2019. Polystyrene nanoplastic exposure induces immobilization, reproduction, and stress defense in the freshwater cladoceran Daphnia pulex. Chemosphere 215: 74-81.
-
31. Lushchak VI. 2011. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101: 13-30.
-
32. Materić D, Kjær HA, Vallelonga P, Tison JL, Röckmann T, Holzinger R. 2022. Nanoplastics measurements in Northern and Southern polar ice. Environ Res 208: 112741.
-
33. Nasser F, Lynch I. 2016. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J Proteom 137: 45-51.
-
34. OECD. 2012. OECD Guideline for Testing of Chemicals, 211 Daphnia magna, Reproduction Test. OECD, Paris.
-
35. Regoli F, Giuliani ME. 2014. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93: 106-117.
-
36. Rhee J-S, Raisuddin S, Lee K-W, Seo JS, Ki J-S, Kim I-C, Park H-G, Lee J-S. 2009. Heat shock protein (Hsp) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants. Comp Biochem Physiol C Toxicol Pharmacol 149: 104-112.
-
37. Rist S, Baun A, Hartmann NB. 2017. Ingestion of micro- and nano- plastics in Daphnia magna-Quantification of body burdens and assessment of feeding rates and reproduction. Environ Pollut 228: 398-407.
-
38. Rossi G, Barnoud J, Monticelli L. 2014. Polystyrene nanoparticles perturb lipid membranes. J Phys Chem 5: 241-246.
-
39. Sies H. 1991. Oxidative stress: from basic research to clinical application. Am J Med 91: S31-S38.
-
40. ter Halle A, Jeanneau L, Martignac M, Jardé E, Pedrono B, Brach L, Gigault J. 2017. Nanoplastic in the North atlantic subtropical gyre. Environ Sci Technol 51: 13689-13697.
-
41. Trevisan R, Ranasinghe P, Jayasundara N, Di Giulio RT. 2022. Nano- plastics in aquatic environments: impacts on aquatic species and interactions with environmental factors and pollutants. Toxics 10: 326.
-
42. Varó I, Perini A, Torreblanca A, Garcia Y, Bergami E, Vannuccini ML, Corsi I. 2019. Time-dependent effects of polystyrene nanoparticles in brine shrimp Artemia franciscana at physio- logical, biochemical and molecular levels. Sci Total Environ 675: 570-580.
-
43. Yoo JW, Cho H, Jeon M, Jeong, CB, Jung JH, Lee YM. 2021. Effects of polystyrene in the brackish water flea Diaphanosoma celebensis: Size-dependent acute toxicity, ingestion, egestion, and antioxidant response. Aquat Toxicol 235: 105821.
-
44. Zhang W, Liu Z, Tang S, Li D, Jiang Q, Zhang T. 2019. Transcriptional response provides insights into the effect of chronic poly- styrene nanoplastic exposure on Daphnia pulex. Chemosphere 238: 124563.