JMLS 2023 June;8(1):56-67. 10.23005/ksmls.2023.8.1.56 Epub 2023 June 16
Copyright © 2023 by The Korean Society of Marine Life Science
Investigation of Enzymatic Activities in Marine Algae-Derived Fungi
Dawoon Chung; Department of Microbial Resources, National Marine Biodiversity Institute of Korea (MABIK), Seocheon 33662, Korea
Woon-Jong Yu; Department of Microbial Resources, National Marine Biodiversity Institute of Korea (MABIK), Seocheon 33662, Korea
Hyeong Seok Jang; Department of Biodiversity, National Marine Biodiversity Institute of Korea (MABIK), Seocheon 33662, Korea
Yong-Min Kwon; Department of Microbial Resources, National Marine Biodiversity Institute of Korea (MABIK), Seocheon 33662, Korea
Seung Seob Bae; Department of Microbial Resources, National Marine Biodiversity Institute of Korea (MABIK), Seocheon 33662, Korea
Grace Choi; Department of Microbial Resources, National Marine Biodiversity Institute of Korea (MABIK), Seocheon 33662, Korea
- Abstract
Marine macroalgae are important in coastal ecosystems and interact with marine microorganisms. In this study, we isolated fungi from seven types of marine macroalgae including Cladophora sp., Gloiopeltis furcate, Gracilariopsis chorda, Hydroclathrus clathratus, Prionitis crispata, Sargassum micracanthum, and Ulva lactuca collected in Korea. Morphological and phylogenetic analyses identified the isolates as four Aspergillus spp. (A. fumigatus, A. sydowii, A. tamarii, and A. terreus), three Penicillium spp. (P. crustosum, P. jejuense, and P. rubens), and Cladosporium tenuissimum. Among them, A. fumigatus TOP-U2, A. tamarii SH-Sw5, and A. terreus GJ-Gf2 strains showed the activities of all enzymes examined (amylase, chitinase, lipase, and protease). Based on the enzymatic index (EI) values in solid media, A. terreus GJ-Gf2 and C. tenuissimum UL-Pr1 exhibited the highest amylase and lipase activities, respectively. Chitinolytic activity was only observed in A. terreus GJ-Gf2, A. tamarii SH-Sw5, and A. fumigatus TOP-U2. Penicillium crustosum UL-Cl2 and C. tenuissimum UL-Pr1 showed the highest protease activities. To the best of our knowledge, this is the first report of lipolytic and proteolytic activities in a marine-derived C. tenuissimum strain. Overall, the fungal strains isolated from the marine macroalgae in this study actively produced industrially important enzymes.
Keywords: Marine algae-derived fungi Amylase Chitinase Lipase Protease
Correspondence to: Grace Choi; Department of Microbial Resources, National Marine Biodiversity Institute of Korea (MABIK), Seocheon 33662, Korea
- Received
- 31 May 2023;
- Revised
- 31 May 2023;
- Accepted
- 3 June 2023.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Language: Korean/English,
Full Text:

Introduction
Fungi are important sources of industrial enzymes that are used in food, feed, cleaning, paper manufacturing, textiles, pharma- ceuticals, and cosmetics (Polizeli et al., 2005). Fungi originating from marine environments have gained increasing attention as producers of enzymes with properties distinct from those of terrestrial microorganisms (Bonugli-Santos et al., 2015). Several marine microbial enzymes exhibit activity that is retained under harsh conditions including high salinity, high pressure, and a wide range of temperatures and pH values (Birolli et al., 2019). For example, Penicillium strain FS010 isolated from the China Yellow Sea produced a cold-adaptive xylanase exhibiting high hydrolytic activities at 2~15℃ (Hou et al., 2006), and polygalacturonases from a marine yeast Cryptococcus liquefaciens strain N6 retain 25~45% of their maximum activity at 0~10°C (Abe et al., 2006). Additionally, chitinase, which originates from the marine fungus Plectosphaerella sp. MF-1, is active at 5℃ (Velmurugan et al., 2011).
Marine fungi have been isolated from diverse substrates includ- ing seawater, sediments, marine animals (fish, mollusks, sponges, etc.), and marine plants (algae, mangrove, etc.). Among these sub- strates, marine algae are not only critical oxygen producers for global ecosystems but also excellent sources of natural products. Macroalgae (seaweeds), which are composed of approximately 25,000~30,000 species, are broadly distributed along the coastline and actively interact with microorganisms (Menaa et al., 2020). Microorganisms including bacteria, fungi, and protists are abundant on the surfaces of diverse macroalgae, and some algae-associated microorganisms produce bioactive compounds with anticancer, antimicrobial, and antioxidant properties (Sarasan et al., 2017).
The cell walls of marine algae contain non-lignocellulosic and sulfated polysaccharides, which are different from those of terres- trial plants (Popper et al., 2011). Because of these unique pro- perties, alga-specific enzymes such as carrageenases, agarases, laminarinases, and alginate lyases have been extensively studied in microorganisms isolated from marine algae (Martin et al., 2014). However, most of these studies have focused on marine bacteria. In addition, other industrially important enzymes such as amylase, protease, and lipases are less elucidated in algae-associated microorganisms relative to the algae-specific enzymes.
In this study, we isolated fungi from a variety of marine macro- algae collected from intertidal zones in Korea. We identified these marine microorganisms via morphological and phylogenetic analyses, and investigated the activities of four widely utilized enzymes: amylase, chitinase, lipase, and protease.
Materials and Methods
1. Sample collection
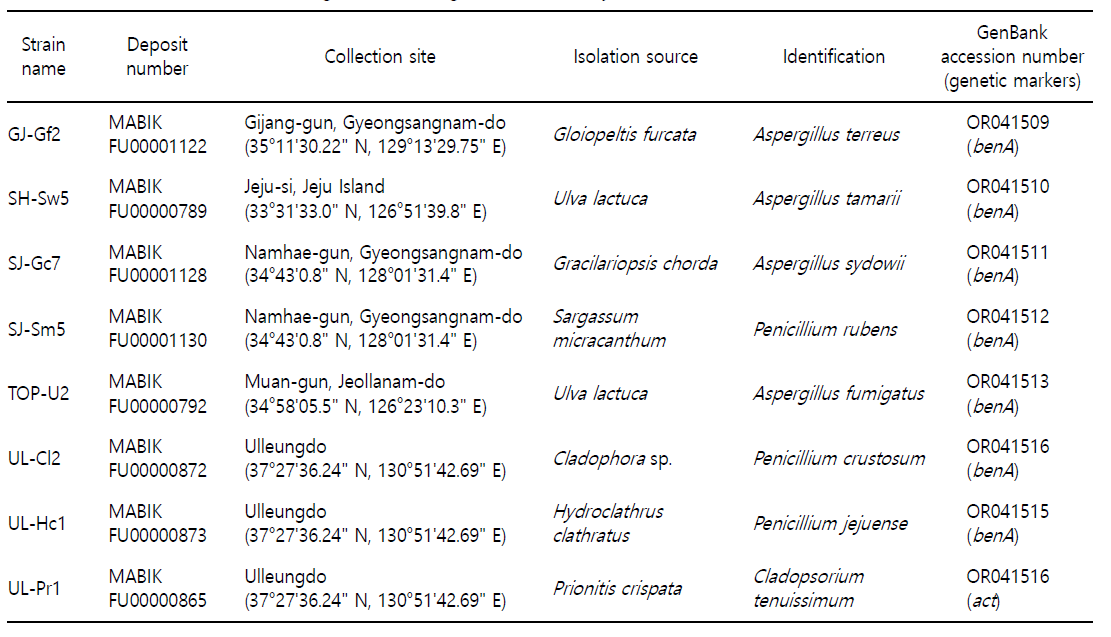
Marine macroalgae were collected from five intertidal zones located in Korea between May 2018 and September 2019. Infor- mation on the isolation sources (macroalgae) and collection sites is presented in Table 1. The algal samples were stored in a thermally insulated box containing ice and transported to the laboratory for fungal isolation.
2. Isolation and cultivation of marine fungi
The collected marine macroalgae (blade parts) were washed several times with filtered local seawater and cut into approxi- mately 1 cm-long pieces using scissors. The cut algae were placed on potato dextrose agar (PDA; BD, Franklin Lakes, NJ, USA) con- taining 3% (w/v) sea salt (Sigma-Aldrich, St. Louis, MO, USA) to culture fungi. All plates were incubated at 20℃ for 7 to 14 days, and individual colonies were transferred to fresh PDA plates repeatedly until obtaining pure cultures. The isolated strains were stored in 20% glycerol at -80℃ and deposited at the Microbial Marine Bio Bank (MMBB) of the National Marine Biodiversity Insti- tute of Korea (MABIK). The MABIK deposit numbers of individual strains are listed in Table 1.
3. Identification of marine fungi
Fungal identification was performed by morphological and molecular analyses. To observe the colony morphology, the fungal strains were grown on PDA at 28℃ for 7 days. To extract genomic DNA (gDNA), fungal strains were cultured in potato dextrose broth (PDB; BD) at 28℃, 200 rpm for 3 days. Fungal tissue preparation and subsequent procedures involving phenol: chloroform: isoamyl alcohol (25:24:1; Sigma-Aldrich) were performed as described previously (Chung et al., 2019).
Polymerase chain reaction (PCR) was conducted to amplify the molecular markers (either the β-tubulin or actin gene, Suppl. Fig. 1) using the primer sets (for β-tubulin, bt2a 5'-GGTAACCAAATCGG- TGCTGCTTTC-3' and bt2b 5'-ACCCTCAGTGTAGTGACCCTTGGC-3'; for actin, ACT-512F 5'-ATGTGCAAGGCCGGTTTCGC-3' and ACT-783R, 5'-TACGAGTCCTTCTGGCCCAT-3'). The thermal cycling condi- tions were as follows: 95℃ for 3 min; 30 cycles of 95℃ for 15 sec, 55℃ for 30 sec, and 72℃ for 1 min; 72℃ for 15 min. PCR pro- ducts were purified using a PCR Purification Kit (Qiagen, Hilden, Germany), and sequencing was performed by Macrogen Inc. (Seoul, Korea). The sequences of the molecular markers of each strain were compared with sequences in the GenBank database using the BLASTN program to identify the closest sequence matches. All sequences were edited and aligned using MEGA version 6 (Tamura et al., 2011), and phylogenetic analysis was con- ducted using the neighbor-joining method with 1,000 bootstrap replicates.

4. Examination of enzymatic activities of marine fungi
The amylolytic activity of the fungal strains was assessed on nutrient agar (BD) containing 0.2% soluble starch (BD) as a sub- strate. Fungal spores (1 × 106 spores in 5 μl) were inoculated on the media and cultured at 28℃ for 5~7 days. After cultivation, the plates were flooded with Lugol's solution (Sigma-Aldrich) for 5 min, drained, and rinsed with distilled water. Amylolytic activity was determined by the presence of a transparent halo around the colonies on dark-purple starch plates.
Chitinolytic activity was assessed as previously described (Chung et al., 2019). Briefly, bacterial suspensions and fungal spores (1 × 106 spores in 5 μl) were inoculated on the media containing 2% colloidal chitin and cultured at 28℃ for 3~7 days. Chitinolytic activity was determined by the presence of a transparent halo around the colonies on opaque colloidal chitin plates.
Lipolytic activity was examined on the media (10 g peptone, 10 g NaCl, 0.1 g CaCl2 · 2H2O, and 15 g Bacto agar (BD) at pH 6) containing 1% (v/v) Tween 20 (Sigma-Aldrich) as a substrate. After cultivation at 28°C for 3~7 days, the activity was determined based on the formation of a precipitate (crystals) around the colonies.
Proteolytic activity was examined in a medium (Czapek-Dox broth (BD), 0.01% (v/v) Triton X-10, and 15 g Bacto agar) con- taining 1% skim milk (BD) as a substrate. After cultivation at 28℃ for 3~7 days, the activity was determined by the presence of a transparent halo around the colonies on opaque skim milk plates.
5. Calculation of enzymatic index (EI)
For comparative analysis of enzyme activities among the fungal strains, the enzymatic index (EI) was calculated as follows:
EI = [diameter of the hydrolyzed zone /diameter of the colonies]
The hydrolyzed zone indicated a halo (amylase, chitinase, and protease) or a precipitate area (lipase). When a fungal colony exhibited an irregular form, the diameter was measured as the mean of the longest and shortest diameters. This experiment was performed in triplicates.
Results
1. Identification of marine algae-derived fungi
Eight fungal strains were isolated from seven types of marine macroalgae: Cladophora sp., Gloiopeltis furcata, Gracilariopsis chorda, Hydroclathrus clathratus, Prionitis crispata, Sargassum micracanthum, and Ulva lactuca (Table 1). On PDA at 28°C, GJ-Sf2, SH-Sw5, and TOP-U2 grew more rapidly than the other five strains (Fig. 1). GJ-Sf2 cells produced brown cinnamon colonies. The SH-Sw5 formed velutinous olive green colonies with floccose tufts. The grayish-green colony of SJ-Gc7 had filiform contours, and the surface was floccose at the center. SJ-Sm5 produced dark green colonies with a velvety surface, whereas UL-Cl2 formed dull-green colonies with corrugation. Both SJ-Sm5 and UL-Cl2 colonies had thin white edges. The UL-Hc1 and UL-Pr1 colonies were grayish-yellow to green and smoke-gray to gray-olivaceous, respectively. The surfaces of UL-Hcl and UL-Pr1 were strongly wrinkled.
Neighbor-joining phylogenetic analysis using the sequences of β-tubulin (benA) or actin (act) identified the marine algae-derived strains as four Aspergillus spp. (A. fumigatus, A. sydowii, A. tamarii, and A. terreus), three Penicillium spp. (P. crustosum, P. jejuense, and P. rubens), and Cladosporium tenuissimum. Individual phylo- genetic trees and genetic markers are shown in Supplementary Fig. 1. Based on the BLASTN search results, the GJ-Gf2 benA sequence was 99.80% and 99.37% identical to A. terreus DTO 438-C8 and NRRL 255 (type strain), respectively (E-value = 0). In addition, in the phylogenetic tree, GJ-Gf2 benA was placed in the same group as A. terreus DTO 438-C8, supported by a 94% bootstrap value. The benA sequence of SH-Sw5 was grouped with those of A. tamarii DTO 418-H8 and NRRL 25593 (bootstrap value 100%). Phylogenetic analyses using the benA sequence assigned SJ-Gc7 and TOP-U2 to the same group as A. sydowii CBS 593.65 and A. fumigatus NRRL 163, respectively (bootstrap values 100%). The SJ-Sm5 benA sequence showed 100% identity to P. rubens DTO:235-E3 and CBS 307.48, and was placed in the same group as the P. rubens isolates, supported by an 86% bootstrap value. Phylogenetic analyses using the benA sequence assigned UL-Cl2 and UL-Hc1 to the same group as P. crustosum CBS 115503 and P. jejuense SFC:P0528, supported by 99% and 98% bootstrap values, respectively. The act sequence of UL-Pr1 showed 100% identity with that of C. tenuissimum CBS 126539 and CBS 125995. In the phylogenetic tree, UL-Pr1 act was grouped with C. tenuissimum CBS 126359, supported by a 93% bootstrap value. The sequences of the genetic markers used for phylogenetic analysis were de- posited in GenBank, and the accession numbers are listed in Table 1.
2. Amylolytic activity of marine algae-derived fungi
All the fungal strains examined exhibited amylolytic activity under the tested conditions (Fig. 2A). Based on the EI values (Fig. 2B), A. terreus GJ-Gf2 showed the highest amylolytic activity (EI value = 1.901 ± 0.022), followed by SJ-Gc7, UL-Pr1, and SJ-Sm5. Although, A. terreus GJ-Gf2 activity was higher than those of A. sydowii SJ-Gc7, C. tenuissimum UL-Pr1, and P. rubens SJ-Sm5 (p < 0.05), SJ-Gc7, UL-Pr1, and SJ-Sm5 did not show significantly different activities (p > 0.05). Only marginal amylolytic activity was observed for A. tamarii SH-Sw5, A. fumigatus TOP-U2, and P. jejuense UL-Hc1.

3. Chitinolytic activity of marine algae-derived fungi
Three Aspergillus spp., A. terreus GJ-Gf2, A. tamarii SH-Sw5, and A. fumigatus TOP-U2, showed chitinolytic activity when colloidal chitin was used as the substrate (Fig. 3A). Because the hydrolyzed zones were observed to be smaller than fungal colonies, we measured the size of the hydrolyzed zones on the bottom of a plate. Based on the EI values, the highest chitinolytic activity was found in A. terreus GJ-Gf2 and A. fumigatus TOP-U2 (EI value 0.667 ± 0.010 and 0.667 ± 0.011, respectively), followed by A. tamarii SH-Sw5 (Fig. 3B). The EI values of GJ-Gf2 and TOP-U2 were not significantly different (p > 0.05), but they were higher than that of SH-Sw5 (p < 0.05).

4. Lipolytic activity of marine algae-derived fungi
Lipolytic activity was examined using Tween 20 as the sub- strate. The highest activity was observed in C. tenuissimum UL-Pr1 (EI value 3.140 ± 0.102), followed by A. sydowii SJ-Gc7 and P. crustosum UL-Cl2 (Fig. 4A). The lipolytic activity of UL-Pr1 was significantly higher than those of SJ-Gc7 and UL-Cl2 (p < 0.05). The activities of A. terreus GJ-Gf2 and A. tamarii SH-Sw5 were not different (p > 0.05). Only marginal lipolytic activity was observed in P. jejuense UL-Hc1 (Fig. 4B).

5. Proteolytic activity of marine algae-derived fungi
When skim milk was used as the substrate, proteolytic activity was observed in all fungal strains examined, except for A. sydowii SJ-Gc7 (Fig. 5A). Both P. crustosum UL-Cl2 and C. tenuissimum UL-Pr1 showed the highest activity (EI values 1.830 ± 0.021 and 1.913 ± 0.032, respectively; p > 0.05), which was significantly higher than P. rubens SJ-Sm5 (EI value 1.163 ± 0.027; p < 0.05). The lowest proteolytic activity was observed for A. terreus GJ-Gf2 (Fig. 5B).

Discussion
Marine macroalgae play critical roles in coastal ecosystems by providing shelter and food for marine organisms. In the present study, we isolated fungi from seven types of marine macroalgae. It is noteworthy that the fungal strains we obtained could be either endophytic (inhabiting algal internal tissues) or epiphytic (inhabiting algal surfaces) because our sample preparation did not involve surface sterilization. Additionally, it is possible that different fungal species could be isolated if distinct algal parts (such as stripes and holdfasts) are used for isolation rather than the blades, as the microbial community might vary depending on the algal parts sampled (Oh et al., 2021).
Among the fungal strains identified in this study, Aspergillus and Penicillium spp. were predominant, accounting for 87.5%. Fungal species of these two genera are ubiquitous and abundant in both terrestrial and marine environments. C. tenuissimum has been reported as a plant pathogenic fungus in several research articles (Nam et al., 2015; Xie et al., 2022). However, C. tenuissimum in the marine environment remains to be characterized.
Among the eight fungal strains tested, A. terreus GJ-Gf2, A. tamarii SH-Sw5, and A. fumigatus TOP-U2 showed activity against all four enzymes. Although amylolytic, chitinolytic, lipolytic, and proteolytic activities are commonly observed in Aspergillus spp. (Farag et al., 2016; Siqueira et al., 2020; Mehta et al., 2020), the chitinolytic activity of A. tamarii has not yet been elucidated. In addition, Cladosporium tenuissimum, which exhibited the highest lipolytic and proteolytic activity in this study, has not been studied for protease production.
The enzymes studied here are widely used and are thus indus- trially important. Proteases are used in the food processing, leather, textile, detergent, and waste treatment industries. They account for more than 65% of enzyme sales worldwide (Shanmugavel et al., 2016). Future work will include the optimization of cultivation and enzymatic reaction conditions (temperature, pH, NaCl con- centrations, etc.) for the maximum activity of each enzyme. These experiments will be critical to determine whether these marine fungi-derived enzymes have the potential for biotechnological applications.
- References
-
1. Abe F, Minegishi H, Miura T, Nagahama T, Usami R, Horikoshi K. 2006. Characterization of cold- and high-pressure active polygalacturonases from a deep-sea yeast, Cryptococcus liquefaciens strain N6. Biosci Biotechnol Biochem 70: 296-299.
-
2. Birolli WG, Lima RN, Porto ALM. 2019. Applications of marine-derived microorganisms and their enzymes in biocatalysis and biotransformation, the underexplored potentials. Front Microbiol 10: 1463.
-
3. Bonugli-Santos R, Vasconcelos MRDS, Passarini MR, Vieria GA, Lopes VCP, Mainardi PH, Dos Santos JA, Duarte LA, Otero IVR, da Ilva Yoshida AM, Feitosa VA, Perssoa Jr A, Sette LD. 2015. Marine-derived fungi: diversity of enzymes and bio- technological applications. Front Microbiol 6: 269.
-
4. Chung D, Baek K, Bae SS, Jung J. 2019. Identification and char- acterization of a marine-derived chitinolytic fungus, Acre- monium sp. YS2-2. J Microbiol 57: 372-380.
-
5. Farag AM, Abd-Elnabey HM, Ibrahim HAH, El-Shenawy M. 2016. Purification, characterization and antimicrobial activity of chitinase from marine-derived Aspergillus terreus. Egyptian J Aquat Res 42: 185-192.
-
6. Hou YH, Wang TH, Long H, Zhu HY. 2006. Novel cold-adaptive Penicillium strain FS010 secreting thermo-labile xylanase isol- ated from Yellow Sea. Acta Biochem Biophys Sin (Shanghai) 38: 142-149.
-
7. Martin M, Portetelle D, Michel G, Vandenbol M. 2014. Micro- organisms living on macrolagae: diversity, interactions, and biotechnological applications. Appl Microbiol Biotechnol 98: 2917-2935.
-
8. Mehta A, Grover C, Bhardwaj KK, Gupta R. 2020. Application of lipase purified from Aspergillus fumigatus in the syntheses of ethyl acetate and ethyl lactate. J Oleo Sci 69: 23-29.
-
9. Menaa F, Wijesinghe PAUI, Thiripuranathar G, Uzair B, Iqbal H, Khan BA, Menaa B. 2020. Ecological and industrial implications of dynamic seaweed-associated microbiota interactions. Mar Drugs 18: 641.
-
10. Nam MH, Park MS, Kim HS, Kim TI, Kim HG. 2015. Cladosporium cladosporioides and C. tenuissimum cause blossom blight in strawberry in Korea. Mycobiology 43: 354-359.
-
11. Oh RM, Bollati E, Maithani P, Huang D, Wainwright BJ. 2021. The microbiome of the reef macroalga Sargassum ilicifolium in Singapore. Microorganisms 9: 898.
-
12. Polizeli MLT, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS. 2005. Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67: 577-591.
-
13. Popper ZA, Michel G, Herve C, Domozych DS, Willats WGT, Tuohy MG, et al. 2011. Evolution and diversity of plant cell walls: from algae to flowering plants. Annu Rev Plant Biol 62: 567-590.
-
14. Sarasan M, Puthumana J, Job N, Han J, Lee JS, Philip R. 2017. Marine algicolous endophytic fungi – A promising drugs resource of the era. J Microbiol Biotechnol 27: 1039-1052.
-
15. Shanmugavel M, Vasatharaj S, Saathiyavimal S, Gnanamani A. 2016. Application of an alkaline protease in biological waste pro- cessing: An eco-friendly approach. Int J Biosci Nanosci. 3: 19-24.
-
16. Siqueira JGW, Torres TMS, Alves BMV, Porto ALF, Porto TS. 2020. Extraction of protease from Aspergillus tamarii URM 4634 in aqueous two-phase system under condinuous and discon- tinuous process. Prep Biochem Biotehcnol 50: 556-563.
-
17. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731-2739.
-
18. Velmurugan N, Kalpana D, Han JH, Cha HJ, Lee YS. 2011. A novel low temperature chitinase from the marine fungus Plec- tosphaerella sp. strain MF-1. Bot Mar 54: 75-81.
-
19. Xie X-W, Huang Y-S, Shi Y-X, Chai A-L, Li L, Li B-J. 2022. First report of Cladosporium tenuissimum causing leaf spots on carnation in China. Plant Dis 106: 1300.